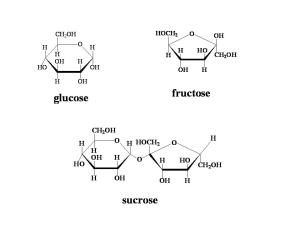
The first topic that I covered in AS Level Biology was about molecules of biological importance. Water is a substance that is in great abundance on this planet, and it holds some significant importance to our lives. Without it, we could not live, and not simply because we would die of thirst. Some of the notes here are also relevant to AS Chemistry, but be careful, as different specifications might ask for information on different anomalous properties, so make sure you check your exam syllabus!
The Properties of Water
- Water is a dipolar molecule:
A water molecule consists of two hydrogen atoms and an oxygen atom; however, the electrons in the covalent bonding are not shared equally. The oxygen atom has a greater electronegativity, meaning that it has a greater pull on the electrons. Due to this each water molecule has slightly negative and slightly positive regions.
- Water molecules form hydrogen bonds:
The negative and positive ends of water molecules attract each other to form hydrogen bonds. These hydrogen bonds give water many of its unique properties. Compounds with molecules similar to the size of water are usually gases. Since each water molecule can form hydrogen bonds with up to 4 other water molecules, water is a liquid at room temperature.
- Water is the universal solvent:
Polar and ionic substances have an electrostatic charge, so they are attracted to the charges on water molecules and dissolve readily. Non-polar substances, such as oil, do not dissolve in water, as they do not have charged molecules. When a salt dissolves in water, the ions separate and a layer of water molecules form around the ions. These layers prevent ions or polar molecules from clumping together, keeping the particles in solution.
- Water has a high surface tension:
At an interface between air and water, a water molecule on the surface forms hydrogen bonds with other molecules around and below it, but not with air molecules above it. The unequal distribution of bonds produces a force called surface tension; this causes the water surface to contract and form a surprisingly tough film or ‘skin’.
Water is at its most dense at 4oC. When water freezes the hydrogen bonds between the molecules forms a rigid lattice, that holds the molecules further apart then in liquid water. Ice, having expanded when freezing, is less dense than its liquid counterpart and so floats on water.
- Water is adhesive and cohesive:
Water is ‘wet’ because it sticks to things. This is because its molecules can form hydrogen bonds with other polar substances. This is called adhesion. The attraction between molecules of similar substances is called cohesion. In this way water molecules stick together which allows water to enter and move along very narrow spaces, in a process called capillarity.
- Important thermal properties:
Water has a high specific heat capacity meaning that it needs to gain a lot of energy to raise its temperature. Conversely it also needs to lose a lot of energy to lower its temperature. Water’s specific heat capacity is 4.2 kJ/g/oC
Water has a high latent heat of vaporisation which means a lot of energy is required to evaporate it. When it evaporates, water draws thermal energy out of the surface it’s on, which can be observed in sweating.
Water also has a high latent heat of fusion meaning that at 0oC water must lose a lot of thermal energy before it freezes, thus liquid water can reach temperatures of down to -10oC before it forms ice.
- Other physical properties of water:
It is transparent to sunlight.
It has a relatively high density compared to air.
It is difficult to compress.
It conducts electricity (when it contains dissolved ions)
See if you can think of ways that these properties are important to life.
An important part of A Level Biology is being able to write a clear and concise essay on the topics you have covered, particularly as many exam boards set an essay question in each of their papers. Below is an example essay on the importance of water.
The Biological Importance of Water
Water has several unique properties that make it vital not only for human beings, but for all living organisms to survive. The most noticeable of its physical properties is that it is a liquid at room temperature, which is unusual for compounds with molecules of a similar atomic composition. This is due to the hydrogen bonds that form between each water molecule, and up to four others. Water being a liquid at room temperature provides a marine environment for organisms to live in, and also provides a liquid environment inside cells, which holds significant importance as metabolic reactions that are key to life take place in solution.
Water molecules are dipolar, meaning they have a positively charged and a negatively charged region. The charges of these areas attract polar and ionic substances that are dissolved in it, and the water molecules form a layer around each charged ion, keeping the substance in solution. Water is known as the ‘universal solvent’, this is because it dissolves much more substances than most common solvents. This is of vital significance as all of the metabolic reactions essential for life take place in solution in the cytoplasm of living cells.
Another property caused by water molecules being dipolar is that water is adhesive, and this adhesion makes water stick to other polar substances, effectively making it ‘wet’. This allows water to move upwards through the very narrow xylem of tall plants, such as trees, against gravity. Continuous columns of water can also be pulled up to the top of trees due to its high tensile strength, meaning that water columns do not break easily. Also important to plants is water’s transparency. Water, being transparent and colourless transmits sunlight, enabling aquatic plants to photosynthesis, and also enabling us to see, as our eyes are coated in water.
There are also many thermal properties that make water so essential for life, for example its very high specific heat capacity, 4.2kJ/g/oC . This means that a lot of energy needs to be gained, or lost, in order to change the temperature of water, and so the environment inside organisms resists temperature changes that could cause it damage. Water also has a high latent heat of vaporisation which means mean that water needs a lot of energy to evaporate, and so draws this thermal energy from the surface it is on, cooling it as the water evaporates from it (this can be observed when we sweat to cool ourselves). Water’s high latent heat of fusion prevents the liquid environment of cells from freezing, and tearing the cells apart, as liquid water temperatures can drop to around -10oC before it begins to freeze.