Continuing on molecules that are important to life, carbohydrates are a huge part of the syllabus which I covered. A comparison of their composition and structure is a common essay title and one which I suggest is learnt. Knowing how to draw the sugar monomers, and also the glycosidic bonds between them, is another important skill.
Carbohydrates:
All carbohydrates contain carbon, hydrogen and oxygen and have the common formula Cx(H2O)y. Carbohydrates include compounds such as sugars, starch, glycogen and cellulose.
Monosaccharides
Simple sugars all have the formula (CH2O)n, meaning that the three component elements are always present in the same ratio. Monosaccharides are grouped according to the value of n, which can range from 3-7.
| n | Sugar |
| 3 | Triose |
| 4 | Tetrose |
| 5 | Pentose |
| 6 | Hexose |
| 7 | Heptose |
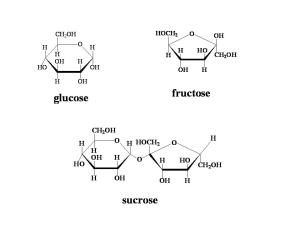
Glucose is the most common hexose, and has the formula C6H12O6. However, this formula does not show how the atoms are structurally arranged, and glucose can have a number of different shapes. Isomerism is when the same atoms are arranged differently to form many shapes. Each compound with the same chemical formula, but different structural formula is called an isomer.
Glucose can form a straight molecule, or two different isomeric rings; alpha glucose and beta glucose. The only difference between the two rings is the position of the hydrogen and hydroxyl groups on carbon atom 1. When the hydrogen atom is above the carbon atom, the ring is called alpha glucose. Beta glucose has the hydroxyl group above the carbon atom.
These different arrangements have very different properties. Alpha glucose molecules combine to form starch, where as beta glucose molecules form cellulose.
Disaccharides
Two monosaccharides can join together to form a disaccharide in a condensation reaction. Different pairs of simple sugars form different disaccharides. The bond formed between the two monosaccharides that joins them is called a glycosidic bond.
| Monosaccharides | Disaccharide formed |
| Glucose + Glucose | Maltose |
| Glucose + Fructose | Sucrose |
| Glucose + Galactose | Lactose |
Condensation involves the removal of water, so ion the process of joining two sugars, one water molecule is formed. The opposite of condensation is hydrolysis, which breaks down the disaccharide into its component part by the addition of water.
Long chains of many monosaccharides joined together can be formed in the same way. A molecule formed of several simple sugars is called a polysaccharide. The process of making polysaccharides is called polymerisation. Polysaccharides are polymers, large molecules formed of multiple repeating units, called monomers. They have the generic formula (C6H10O5)n. Polysaccharides are not particularly soluble in water, are not sweet, and do not form crystals. The properties of polysaccharides are determined by the number and type of the monomers joined together, and also the shape that they form. Polysaccharides can be straight, helical, coiled or branched.
Each carbon atom in a monosaccharide unit is given a number, (in glucose from 1-6), different carbon atoms bond in different ways to form different chains of polysaccharides. The reaction between the hydroxyl groups at carbon atom 1 of one molecule, and atom 4 of another molecule forms a 1-4 glycosidic bond, many of which form long, straight chains. Branched chains have one or more 1-6 glycosidic bonds, which form between the hydroxyl groups on a carbon atom 1 and a carbon atom 6.
Starch is formed of hundreds of repeating alpha glucose molecules. It is compact due to its tight globular structure. Its natural spiral structure is compressed due to the attraction between the different molecules. When an Iodine molecule is trapped in the spiral of the starch, it changes colour from brown to blue/black, which is what we see when testing for starch in food tests. In plants, starch is stored in the membranous organelles called plastids, e.g. chloroplasts. Starch is a source of organic carbon for making other substances and is also broken down during digestion into glucose for respiration.
Another storage polysaccharide is glycogen, it is found mainly in the liver and muscle cells. It has a similar roles and structure to starch, and since it is more abundant in animals, it is often referred to as ‘animal starch’. Glycogen is less dense and more soluble than starch as it has many more 1-6 glycosidic bonds. It can be broken down (hydrolysed) more readily than starch, as such animals have a higher metabolic rate than plants.
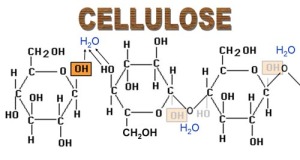
Cellulose is another polysaccharide, and it is a major component of plant cell walls. It is formed by beta glucose monomers linked by 1-4 glycosidic bonds. Cellulose is completely permeable, meaning that it allows water and other substances to pass through it into and out of the cell freely. Unlike starch and glycogen, cellulose cannot be hydrolysed easily. Animals such as cows are able to digest plant matter efficiently as their stomachs produce cellulase, an enzyme that speeds up the breakdown of cellulose. Since humans do not produce cellulase, we cannot obtain the nutrients contained in plant cells. Lingin is used by plants to strengthen cellulose further. It creates an impermeable lining on the xylem tubes, providing added support, and also helps prevent infection and decay.